A method that may help to elucidate the overall question whether quantitative chimerism analysis can serve as a surrogate MRD-marker in the posttransplant period
Dresden, 23rd of Feb, 2016
Allogeneic stem cell transplantation (HSCT) is an effective therapy for hematopoietic disorders such as Acute Myeloid Leukemia (AML) or the Myelodysplastic Syndrome (MDS). However, imminent relapse remains a serious problem. Routinely, monitoring of minimal residual disease (MRD) by diseases-specific markers such as FLT3, NPM1, BCR-ABL, AML-ETO and others is performed to signify threatening relapse as early as possible. However, a high number of patients lack specific disease-markers. For such patients quantitative chimerism monitoring in the posttransplantation period is widely discussed to serve as surrogate MRD-marker, representing a prognostic factor that predicts the clinical outcome.
Biotype Diagnostic developed a quantitative diagnostics for chimerism monitoring after Allo-HSCT. By the combination of two established methods, Biotype has engineered a product that allows engraftment control along with quantitative chimerism monitoring. Detecting only 1 – 5 diseased blood cells in 10.000 healthy cells this new product increases the sensitivity by a factor of 20 compared to standard methods.
Biotype’s DIPscreen/DIPquant strategy for chimerism analysis deploys Insertion/Deletion DNA-polymorphisms (INDELs) that allow an allele-specific quantification by qPCR. More than 50 assays are available that were shown to accomplish a detection limit lowered to 0.05 % sensitivity. The DIP-marker assortment was strictly led by scientific data and bioinformatics and can be predicted to be sufficiently informative to analyse > 90 % of donor/recipient pairs.
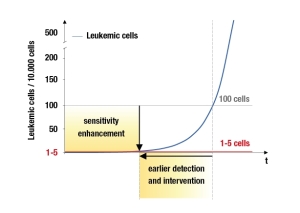
Together, this analysis reflects a new and promising strategy to reliably and quantitatively monitor the post-transplant progress. Based on highly accurate information it supports decision making and provides a quantitative monitoring opportunity for leukaemia patients lacking common disease-specific markers. This method may therefore help to elucidate the overall question whether quantitative chimerism analysis can serve as a surrogate MRD-marker in the posttransplant period.
About Biotype Diagnostic
Biotype Diagnostic GmbH is a manufacturer of molecular in-vitro diagnostics (IVD). The company represents high expertise for orphan-diseases and addresses medical areas such as haematology/oncology with highly specific and customized applications. The company draws back on more than 15 years of experience in developing DNA-based analysis and stays in close collaboration with academic and clinical partners to continuously transfer scientific know-how into marketable products.
Biotype Diagnostic GmbH
Dr. Sophia Mersmann
Moritzburger Weg 67
01109 Dresden
Germany
Tel.: + 49 0351 8838 432
s.mersmann@biotype.de
www.biotype.de